 |
 |
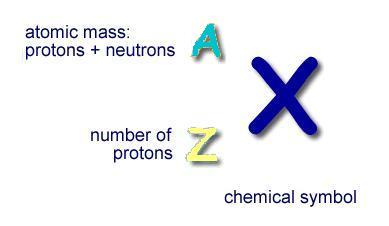
- The illustration below shows the chemical symbol for the hypothetical element "X".
- The number of protons in the nucleus is represented by "Z", the atomic number
- All the isotopes of an element have the same "Z"
- The atomic mass of the element (number of protons plus the number neutrons) is represented by "A"
- "A" is usually place to the left above the element symbol
- The number of neutrons in the nucleus is equal to A minus Z
- Two different forms, or isotopes, of carbon are shown below:
- Carbon-12: with 6 protons and 6 neutrons and an atomic mass of 12
- Carbon-14: with 6 protons and 8 neutrons, and an atomic mass of 14
Adapted from
Understanding Radiation (EPA)
|





