About Dicentric Chromosome Assays
Warning: cautions and limitations
- Few laboratories have sufficient experience with the rigorous quality control and dose calibration necessary to perform this dicentric chromosome assay when it is used to estimate radiation absorbed dose.
- REMM specifically recommends against clinicians using results from non-reference laboratories for making clinical decisions in a radiation emergency.
- Dicentric Chromosome Assay results reported from radiation reference laboratories will include an estimated radiation dose, not just a count of dicentric chromosomes.
- This result can be useful in clinical decision-making along with other clinical (signs and symptoms) and laboratory data in order to triage and manage patients with whole body or partial body radiation exposure.
(See exposure algorithm)
- This result can be useful in clinical decision-making along with other clinical (signs and symptoms) and laboratory data in order to triage and manage patients with whole body or partial body radiation exposure.
- Major US government laboratories with experience performing radiation emergency related dicentric chromosome assays include
- Oak Ridge Institute for Science and Education,
Radiation Emergency Assistance Center/Training Site (REAC/TS)
Cytogenetic Biodosimetry Laboratory
orise.orau.gov/reacts/cytogenetic-biodosimetry-laboratory.html
Attention: Dr. Adayabalam Balajee
P.O. Box 117, MS 39
Oak Ridge, TN 37831-0117
REAC/TS office phone numbers and for biodosimetry specimens
- Day hours, Monday-Friday: 865-576-3131
- After hours, weekends, and emergencies: DOE Oak Ridge Operations Center (OROC): 865-576-1005 (Ask for REAC/TS)
- Armed Forces Radiobiology Research Institute (AFRRI)
afrri.usuhs.edu
8901 Wisconsin Avenue
Bethesda, MD 20889-5603
Emergency Contact Number:
1-301-295-0530
AFRRI must be contacted directly before sending any specimens
- Oak Ridge Institute for Science and Education,
- In a large mass casualty incident, it will be difficult for these 2 laboratories to handle the surge capacity for the entire nation.
- See also:
- Dicentric Chromosome Assay as a Biodosimetry tool: Utility and Limitations
- Four References Comparing Biodosimetry Tools
- Romm H, Beinke C, Garcia O, Di Giorgio M, Gregoire E, Livingston G, Lloyd DC, Martìnez-Lopez W, Moquet JE, Sugarman SL, Wilkins RC, Ainsbury EA. A New Cytogenetic Biodosimetry Image Repository for the Dicentric Assay. Radiat Prot Dosimetry. 2016 Dec;172(1-3):192-200. [PubMed Citation]
Background information
- Illustrations of normal chromosome and dicentric chromosome
- Dicentric Chromosome Assay is based on the principle that radiation exposure causes DNA strand breaks, in particular double strand breaks.
- During repair of DNA strand breaks, misrepair of 2 chromosomes and abnormal chromosome replication can lead to dicentric chromosomes, that is, a chromosome with 2 centromeres.
- Increases in radiation dose produce increasing numbers of dicentric chromosomes.
- Although radiation induces many types of chromosomal changes in addition to dicentric chromosomes, dicentrics are considered the most sensitive and most specific for assessing radiation dose.
- Factors that influence the results of Dicentric Chromosome Assay include
- Total dose
- Dose rate
- Percent body irradiated (whole vs. partial body)
- Radiation quality (e.g., photon vs. neutron)
- Sampling time after exposure
- Optimal time for sample collection: between 24 hours and about 4-6 weeks post-exposure
- Specimens drawn after 6 weeks can be analyzed using time correction factors.
- Dicentric Chromosome Assay is
- Time consuming and highly technique dependent
- Very specific for radiation
- Background level for dicentrics in the population is low, about 1 dicentric chromosome in 1,000 cells
- Few chemicals (except those which are "radiomimetics") cause dicentrics.
- Very sensitive for radiation
- Threshold dose as low as about 0.05 Gy may be identified by this assay.
- There is a good dose-effect relationship in the range of 0.05 Gy to 5.0 Gy for acute exposures to low linear energy transfer (LET) radiation
- The Dicentric Chromosome Assay is both specific and sensitive for radiation exposure even at low doses.
- Use of this assay after a large radiation mass casualty emergency
- Senior emergency managers will provide information about approved reference laboratories available to analyze specimens.
- Dose estimation from the Dicentric Chromosome Assay, even from reference laboratories, should be used in conjunction with all other clinical (signs and symptoms) and laboratory information in order to triage and manage patients with significant radiation exposure.
- Clinicians may have difficulty using this assay in the initial phase of a large incident because
- There is limited national laboratory capacity.
- Time to receive assay results is typically at least 3-4 days after a sample is received.
- Patient who receive high doses usually require management decisions before the results are available.
- Overview of methods
- Peripheral blood is obtained from individuals potentially exposed to radiation.
- Lymphocytes are stimulated to grow in culture and cells in first-division metaphase spreads are collected on glass slides.
- Cells with chromosomes in the metaphase stage of division are stained and analyzed for chromosome aberrations.
- Dose estimates are made by
- Counting the frequency of dicentrics in the sample
- Comparing the frequency of dicentrics counted to calibration curves reflecting radiation quality, e.g., 250 kVp x rays, fission neutrons, and Cobalt-60 gamma rays 2 (See Figure)
- Significant laboratory expertise with performing the Dicentric Chromosome Assay for clinical use in dose estimation is crucial.
- Reference laboratory inter-comparisons for quality assurance should be done periodically.
- Calibration curves can vary slightly even among reference laboratories.
- An important study comparing results from multiple reference laboratories has been published. 3
- Scoring 5
- Metaphase counting (scoring) of dicentrics is performed on a representative sample of cultured lymphocytes.
- As few as 20 metaphases from peripheral blood lymphocytes may indicate confirmation of an exposure.
- Counting 20 metaphases facilitates more rapid analysis, as after a mass casualty event.
- Scoring 50 metaphases provides a more accurate result than scoring 20.
- Where there is disagreement with initial assessments or evidence of significantly inhomogeneous exposure, resolution may be provided by increasing the number of scored metaphases from 20 to 50, and then to as many as 500 or 1,000. Scoring this large number requires a significantly longer period of time.
- Scored metaphase samples showing an inhomogeneous number of dicentrics per cell provides evidence for partial-body exposure. 4
- Metaphase counting (scoring) of dicentrics is performed on a representative sample of cultured lymphocytes.
- When to draw blood for the dicentric chromosome assay 5
- Optimal time is at least 24 hours after exposure.
- Dicentrics are formed by a misrepair of 2 chromosomes
- 24 hours allows sufficient time to permit completion of repair by lymphocytes
- For suspected doses > 4 Gy, AFRRI suggests drawing blood at 1-6 hours post-event in order to obtain a sufficient population of lymphocytes. After 6 hours the peripheral blood may be depleted of lymphocytes.
- NOTE: Drawing blood 1-6 hours after an exposure may not be possible in a mass casualty situation.
- Samples drawn within 4-6 weeks after exposure
- Valid results do not require correction for sampling delay relative to the half-life of circulating lymphocytes in peripheral blood.
- Samples drawn >6 weeks after exposure
- Valid results require application of a correction factor to account for the lymphocyte life span in circulating blood.
- Unstable aberrations (primarily dicentric and ring forms) are eliminated over time, decreasing assay sensitivity.
- Samples drawn between 6 - 12 months after exposure
- Can be used to estimate doses > 0.5 Gy (acute photon exposure), if appropriate corrections are made.
- Optimal time is at least 24 hours after exposure.
- Use of dicentric chromosome assay in acute emergency triage and management
- Victims with a potential whole-body dose in the range of 1.5-3 Gy
- May be at risk for the ARS hematological subsyndrome.
- May develop cytopenias about 21-28 days after exposure.
- Dicentric Chromosome Assay may help identify persons who should be triaged to locate near a medical facility having expertise in managing pancytopenic patients.
- Victims with potential whole-body doses >3 Gy
- May become symptomatic and pancytopenic before the results of dicentric chromosome assay become available. Lymphocyte depletion kinetics are useful in this setting.
- Nevertheless, Dicentric Chromosome Assay results could provide useful confirmatory information.
- Risk assessment for carcinogenicity
- Although there are currently no known effective interventions to prevent radiation-induced cancer following high-dose radiation exposure, an estimate of the dose received may be useful in providing guidance about subsequent monitoring and follow-up over months to years.
- See REMM information about the
- Victims with a potential whole-body dose in the range of 1.5-3 Gy
- What other chromosomal assays may be helpful?
- Radiation-induced chromosomal translocations can be detected with the fluorescent in situ hybridization (FISH) technique as an alternative to the dicentric chromosome assay in specific circumstances 6
- For retrospective dose assessment when blood is drawn long after the exposure (e.g., > 1 year), FISH is thought to be more accurate than the dicentric chromosome assay.
- FISH measures cell division compatible translocations (symmetrical translocations).
- Translocations in the stem cell population are transmitted through lymphopoiesis, ultimately appearing in the circulating pool of lymphocytes.
- However, several confounding factors (including a high background incidence, variability among individuals, non specificity) make the FISH assay less attractive for acute radiation dose assessment.
- See other techniques (including premature chromosome condensation (PCC) and the micronucleus assay) and detailed information in the references list
- See excellent table comparing advantages and disadvantages of existing and developing techniques, including the dicentric chromosome assay, for assessment of radiation exposure levels. 7
- Radiation-induced chromosomal translocations can be detected with the fluorescent in situ hybridization (FISH) technique as an alternative to the dicentric chromosome assay in specific circumstances 6
- Are there standards published for chromosomal assays for radiation dose assessment?
Illustrations
Dicentric Chromosomes
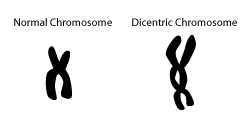
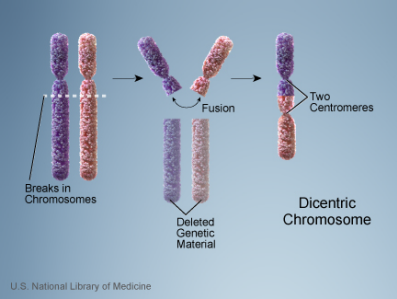
Notes on the graphs above:
- Radiation exposure causes DNA strand breaks leading to abnormal chromosome replication, including dicentric chromosome formation. This figure illustrates the double-strand break.
- Increases in radiation dose produce increasing numbers of aberrant (dicentric) chromosomes.
- The number of dicentric chromosomes correlates well with radiation dose (i.e., increases in dose produce increased numbers of dicentric chromosomes).
Conventional Lymphocyte Metaphase-Dicentric Assay
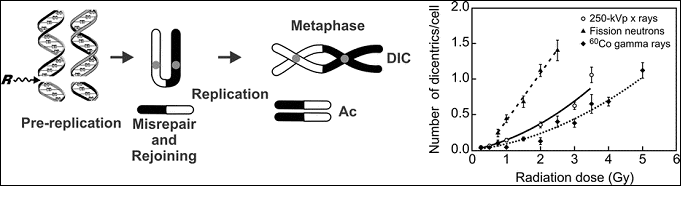
Notes on the graph above:
- Left panel: A schematic illustrating radiation-induced DNA damage (see left side of image) in an interphase cell and the resulting formation of radiation-induced dicentric (DIC) and accompanying acentric fragment (Ac) chromosome aberration in lymphocytes arrested in metaphase mitosis.
- Right panel: Dose response relationship for dicentrics in human lymphocytes exposed to incremental radiation doses from three radiation qualities: 250 kVp x rays, fission neutrons, and Cobalt-60 gamma rays.
- Prasanna PGS, Loats H, Gerstenberg HM, et al. AFRRI's gamma-ray, x-ray and fission-neutron calibration curves for the dicentric assay: Application of a metaphase finder system. (PDF - 309 KB) Fort Belvoir, VA, USA; AFRRI, Defense Technical Information Center, Fort Belvoir, VA, 2002
Scoring of Dicentrics
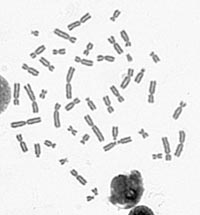
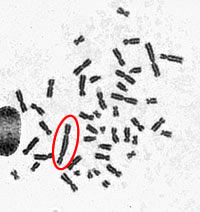
- A digital photo of the metaphase spread is taken at 630x magnification.
- A dicentric chromosome has two centromeres — the point where the two chromatids connect.
- The number of dicentric chromosomes is counted and compared to calibration curves to estimate radiation dose.
- Image A displays a normal metaphase spread.
- Image B reveals a metaphase spread with a dicentric chromosome circled in red.
- Cytogenetic Biodosimetry Laboratory (Radiation Emergency Assistance Center/Training Site (REAC/TS))
References
- Romm H, Beinke C, Garcia O, Di Giorgio M, Gregoire E, Livingston G, Lloyd DC, Martìnez-Lopez W, Moquet JE, Sugarman SL, Wilkins RC, Ainsbury EA. A New Cytogenetic Biodosimetry Image Repository for the Dicentric Assay. Radiat Prot Dosimetry. 2016 Dec;172(1-3):192-200. [PubMed Citation]
- Prasanna PGS, Loats H, Gerstenberg HM, et al. AFRRI's gamma-ray, x-ray and fission-neutron calibration curves for the dicentric assay: Application of a metaphase finder system. (PDF - 309 KB) Fort Belvoir, VA, USA; AFRRI, Defense Technical Information Center, Fort Belvoir, VA, 2002
- Wilkins RC, Romm H, Kao TC, Awa AA, Yoshida MA, Livingston GK, Jenkins MS, Oestreicher U, Pellmar TC, Prasanna PG. Interlaboratory comparison of the dicentric chromosome assay for radiation biodosimetry in mass casualty events Radiat Res. 2008 May;169(5):551-60. [PubMed Citation]
- Prasanna PG, Moroni M, Pellmar TC. Triage dose assessment for partial-body exposure: dicentric analysis. Health Phys. 2010 Feb;98(2):244-51. [PubMed Citation]
- Prasanna PGS: Personal communication
- Fucic A, Zeljezic D, Kasuba V, Kopjar N, Rozgaj R, Lasan R, Mijic A, Hitrec V, Lucas JN. Stable and unstable chromosome aberrations measured after occupational exposure to ionizing radiation and ultrasound. Croat Med J. 2007 Jun;48(3):371-7. [PubMed Citation]
- High dose radiation effects and tissue injury, Report of the Independent Advisory Group on Ionising Radiation, Table 4.4, page 41 (Public Health England [PHE], formerly Health Protection Agency [HPA], UK)
- Videos:
- Dicentric Chromosome Assay (Vimeo: 1:44 min) (REAC/TS)

- Cytogenetic Biodosimetry Laboratory (YouTube: 5:10 min) (REAC/TS)

- Dicentric Chromosome Assay (Vimeo: 1:44 min) (REAC/TS)
See also:
- Dose Estimator for Exposure
- Biodosimetry References List
- REMM Biodosimetry Reference List in the section entitled Dicentric Chromosome Assays as a Biodosimetry Tool. Other types of chromosome assays are also listed.
- Biodosimetry Reference List: graphic listing

